Bilateral Modified Radical Neck Dissection for Metastatic Papillary Thyroid Carcinoma
Abstract
Radical neck dissection was once the standard of care for the surgical management of patients with thyroid cancer and cervical lymph node metastases. However, due to the significant morbidity of this procedure, the development of cervical lymphadenectomy procedures that could provide oncologic cure while minimizing morbidity was undertaken by many surgeons. Such an investigation has led to the development of the modified radical neck dissection (MRND). Still, many institutions are not familiar with performing a comprehensive MRND in the setting of thyroid cancer metastatic to the lateral lymph node compartments. We present such an operation under general anesthesia.
Keywords
Cervical lymphadenopathy; cervical lymph node metastases; modified radical neck dissection.
Case Overview
Background
Thyroid cancer is the most common endocrine malignancy in the U.S. The rate of new cases has been steadily rising over the past several decades, with the vast majority being papillary thyroid carcinomas (PTC).1,2 The most common route of spread of PTC is to cervical lymph nodes; up to 40–90% of patients with PTC have evidence of cervical lymphadenopathy at the time of diagnosis.3
Focused History of Patient
The patient is a 61-year-old male who presents with bilateral persistent metastatic PTC to the bilateral lateral neck lymph node compartments. In 2014, he had undergone thyroid cancer screening after his brother and sister were found to have papillary thyroid cancer. At an outside institution, he had a total thyroidectomy in March 2014, at which time the right thyroid PTC was noted to have adhered to the right recurrent laryngeal nerve. The final pathology showed bilateral PTC with the largest tumor measuring 3.1 cm on the left side with extrathyroidal extension. 3 of 6 lymph nodes contained PTC. Preoperatively, he had undergone a CT scan of the neck that clearly demonstrated bilateral lateral neck lymphadenopathy consistent with metastatic disease (Figure 1). However, the lateral neck disease was, for unknown reasons, never addressed at the initial operation. He had trouble with his voice after surgery and still had issues with voice fatigue postoperatively. He was treated with radioactive iodine 134mCi in April 2014. He had no evidence of metastatic disease on the post-treatment iodine scan, but thyroglobulin levels were persistently elevated.
His course was unremarkable for the initial years, but then in 2017, an ultrasound showed bilateral level III lymph nodes with calcifications. They were possibly slightly enlarged compared with previous imaging, but thyroglobulin levels were unchanged. In April 2018, he underwent a comprehensive ultrasound with lymph node mapping, demonstrating lymph nodes concerning for metastatic PTC in bilateral lateral neck compartments (i.e. lymph node compartment levels II–V). At this time, the thyroglobulin level was elevated at 48, with a thyroglobulin antibody titer of less than 10. Fine needle aspiration (FNA) biopsies of the right level IV and left level III lymph nodes were performed, and both were positive for PTC, proving that the patient had bilateral recurrent metastatic PTC to the bilateral lateral neck lymph node compartments. In addition, CT scans of the neck and chest with and without IV contrast were also consistent with these findings. At this time, he was referred to Yale Endocrine Surgery for consideration of remedial surgery. There were no other complaints of hoarseness, difficulty swallowing, or breathing.
The biochemical evaluation demonstrated a normal TSH of 1.13, elevated thyroglobulin at 48 (unstimulated; without positive thyroglobulin antibodies), and total serum calcium of 9.4 mg/dl (reference range 8.8-10.2 mg/dl).
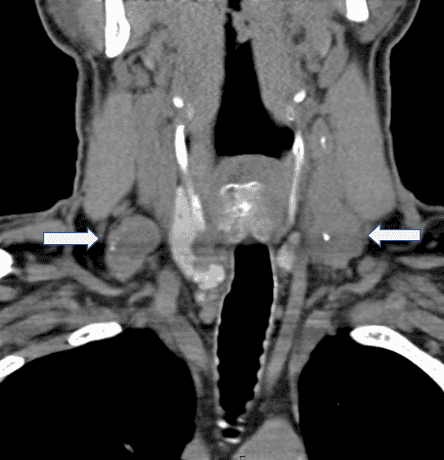
Figure 1. CT scan of the neck from 2014.
Positive lymphadenopathy in the bilateral cervical lymph node compartments (arrows).
Unfortunately, these were not resected at the initial operation performed at an outside institution.
Imaging Studies
A comprehensive ultrasound with lymph node mapping was performed, demonstrating lymph nodes concerning for metastatic PTC in bilateral lateral neck compartments (i.e. lymph node compartment levels II–V). Fine needle aspiration (FNA) biopsies were performed of the largest right level IV (measuring 2.8 x 1.9 x 1.8 cm, with extension into the level V lymph node compartment) and left level III lymph nodes (measuring 3.7 x 2.1 x 2.9 cm, with extension into the level V lymph node compartment, as well), and both were positive for PTC.
He underwent a CT scan of the neck and chest, which demonstrated abnormal lymph nodes in the neck soft tissues, some demonstrating pathological enlargement, calcifications, and central necrosis, concerning for metastatic disease. Representative examples included a 3-cm left level III lymph node, which narrows the adjacent internal jugular vein, a 2.1-cm right level 3 lymph node, a 9-mm left level II/III lymph node anterior to the carotid space, with possible lymph node abutting the bifurcation of the right subclavian and right internal jugular veins, and a 1.7-cm left level IV/supraclavicular lymph node.
Natural History
The presence of cervical lymph node metastases in thyroid cancer portends a decreased survival rate as compared to its absence.4 Accordingly, lateral neck lymph node dissection remains a mainstay in the surgical treatment of this disease process.
Options for Treatment
Surgery is the only primary treatment for metastatic PTC. However, adjuvant radioactive iodine therapy (RAI) is often instituted to treat persistent microscopic disease, as well as ultrasound-guided alcohol ablation in select cases.5 Occasionally, when surgery is not possible, palliative external beam radiation therapy is used.
Discussion
Radical neck dissection (RND) was first described by George Crile in 1906 and has remained the standard of care for the treatment of cervical metastases in head and neck squamous cell cancers.6 The standard procedure involves the removal of lymph nodes in all compartments of the lateral neck (levels I through V), as well as resection of the spinal accessory nerve (SAN), internal jugular vein (IJV), and sternocleidomastoid muscle (SCM). The procedure is associated with substantial long-term disability, including shoulder dysmotility and cosmetic deformity.7 Because of the significant morbidity of this procedure, efforts have been undertaken to provide alternative surgical approaches that are equally efficacious but less morbid than the standard RND.
Two alternative procedures that have been developed include the modified radical neck dissection (MRND) and selective neck dissection (SND). Modified radical neck dissection involves the removal of all lymph nodes typically removed in the RND, with sparing or preservation of at least one of the following structures: SAN, IJV, SCM. A selective neck dissection refers to a cervical lymphadenectomy in which there is the preservation of one or more of the lymph node groups that are routinely removed in the radical neck dissection. To date, no prospective randomized trials comparing RND to either MRND or SND exist. However, there are several retrospective studies comparing outcomes after RND versus MRND; mean combined recurrence rates were significantly lower in the MRND groups [6.9%, 95% confidence interval, 5.4–8.4%] as compared to the RND groups [13.6%, 95% confidence interval, 12.0–15.2%].8
In the setting of metastatic PTC, cervical lymphadenopathy remains the most common source of disease recurrence or persistence.9 Therefore, adequate cervical lymphadenectomy is a primary determinant of outcomes after surgery. However, significant controversy remains regarding the extent of lateral neck dissection that is necessary to achieve optimal outcomes. A study by Caron et al.10 suggested that there is a much higher incidence of lymph node involvement of levels III and IV, and therefore SND is adequate in most cases of metastatic PTC. One criticism of this study is that only a small number of patients were included. We recently demonstrated in an extensive series of MRND procedures at a single institution that omitting levels II and V potentially leaves behind the disease in 67% and 20% of patients, respectively; therefore, MRND is the optimal procedure to minimize the morbidity associated with reoperative surgery for missed disease.11
Surgical Technique and Results
Surgeons performing MRND must have a thorough understanding of the anatomy of the neck, as well as embryology of the parathyroid glands. In general, MRND is indicated whenever preservation of the SAN, SCM, or IJV is possible without compromising a complete oncologic resection.
A transverse (Kocher) incision is created and extended laterally along the lateral neck. In this particular patient, the previous scar was excised to ensure the best possible cosmetic outcome. Subplatysmal flaps are elevated superiorly and inferiorly. The greater auricular nerve is identified and then followed to Erb's point (it is located on the posterior border of the sternocleidomastoid muscle, approximately 2–3 cm above the clavicle, where the greater auricular, lesser occipital, transverse cervical, and supraclavicular nerves emerge. It is a critical landmark for identifying the spinal accessory nerve) and is preserved. Approximately 1.5 cm above Erb's point, the SAN is identified. The SAN is then carefully dissected out and preserved as it enters the trapezius muscle. As the SAN exits the anterior triangle and enters the posterior triangle, it crosses over the GAN under the surface of the sternocleidomastoid muscle forming an imaginary cross (“X-pointer”). At the posterior border of the SCM, the SAN is found superior to the GAN and this relationship gets reversed at the anterior border. The cervical rootlets often have a similar course as that of the accessory nerve and can confuse the surgeon. In this context, X-pointer is an additional dependable, constant and easy landmark to differentiate the SAN from the other nerves; it is independent of body proportions.12 Other landmarks for SAN identifications are the followings:
- The SAN in relationship to the IJV is coursing dorsally in 44% and ventrally in 56% cases (Kierner et al.).
- The contribution of the cervical plexus to the SAN is primarily from the C2, C3, and a combination of the two and has communication to the nerve deep to the SCM muscle in the posterior triangle.
- Shiozaki et al. reported three types of innervations to the SCM muscle classified as type A—non-penetrating type, type B—partially penetrating type, and type C—completely penetrating type.
- The mean distance from the midpoint of the clavicle to the point of entry of the SAN into the trapezius muscle is about 59 mm. This is an important landmark for tracing the SAN from the lower neck and dissecting superiorly. The SAN innervated all parts of the trapezius muscle, and hence, the isolation and preservation of the nerve became important.13
The level V lymph node compartment is then mobilized from lateral to medial. The entire cervical fat pad encompassing the level V lymph nodes is dissected free. The external jugular vein is divided superiorly and inferiorly. The fascia is then unwrapped from the SCM. The omohyoid muscle is dissected out and preserved; if necessary for better exposure, it can be sacrificed. The IJV, common carotid artery, and vagus nerve are dissected free and preserved throughout. The specimen is then mobilized from lateral to medial, encompassing all the fibro-fatty lymph node-bearing tissue from levels V, IV, III, and II. Dissection is carried superiorly to the digastric muscle. The glossopharyngeal nerve is identified and preserved. Dissection is carried inferiorly to below the clavicle. In the case of a left-sided lateral dissection, the thoracic duct is identified and preserved; it can also be ligated if necessary. Although there is no dominant lymphatic duct on the right side, care should still be taken during the supraclavicular dissection to avoid a lymphatic/chyle leak. The phrenic nerve is identified medially. It lies lateral to the vagus nerve, and the brachial plexus is identified laterally to the phrenic nerve; both are preserved. Phrenic nerve is an important landmark as it innervates the diaphragm, following the prevertebral fascia during dissection would be helpful for preventing the injury to phrenic nerve. Small branches of the transverse cervical nerves are divided as necessary. The entire specimen is then removed and sent to pathology for the permanent section.
In this particular patient, the cancer significantly adhered to the omohyoid muscle as well as the internal jugular vein in the left level IV lymph node compartment. Despite the well known margins of level IV (anterior margin is lateral border of the sternohyoid muscle, posterior border is the posterior border of SCM, superior border is omohyoid muscle and inferior border is clavicle) the operating surgeon thus resected the internal jugular vein and omohyoid muscle en bloc to achieve negative margins. A surgical drain (#10 Jackson-Pratt) was placed on each side of the neck.
Pathology and Follow-Up
The surgical pathology revealed that the scar revision only contained benign skin with scar. In total, 10 of 34 and 5 of 26 lymph nodes were positive in the left and right MRND specimens, respectively. All lateral neck lymph node compartments (levels II–V) contained at least 1 positive lymph node except for the left level II lymph node compartment. The largest positive lymph node on the left side measured 3.9 cm, and on the right side 3.8 cm. There was evidence of extranodal extension of the PTC in the bilateral lateral neck. At approximately 10 days postoperatively, his bilateral cranial nerves (CN) were all grossly intact (CN II–XII) and his vocal cord function as assessed by flexible laryngoscopy was completely normal. Additionally, his serum calcium was normal at 9.4 mg/dl [reference range 8.8–10.2 mg/dl].
Equipment
No special equipment was used.
Disclosures
Nothing to disclose.
Statement of Consent
The patient referred to in this video article has given their informed consent to be filmed and is aware that information and images will be published online.
Citations
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review (CSR) 1975-2016. Bethesda, MD: National Cancer Institute. Available at: http://seer.cancer.gov/csr/1975_2016/.
- Cooper DS, Doherty GM, Haugen BR, et al.; American Thyroid Association Guidelines Taskforce. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16(2): 109-142. doi:10.1089/thy.2006.16.109.
- Carling T, Udelsman R. Thyroid cancer. Annu Rev Med. 2014;65:125-137. doi:10.1146/annurev-med-061512-105739.
- Jin S, Bao W, Yang YT, Bai T, Bai Y. Establishing a prediction model for lateral neck lymph node metastasis in patients with papillary thyroid carcinoma. Sci Rep. 2018;8(1):17355. doi:10.1038/s41598-018-35551-9.
- Suh CH, Baek JH, Choi YJ, Lee JH. Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid. 2016;26(3):420-428. doi:10.1089/thy.2015.0545.
- Crile G. Excision of cancer of the head and neck. With special reference to the plan of dissection based on one hundred and thirty-two operations. JAMA. 1906;XLVII(22):1780-1786. doi:10.1001/jama.1906.25210220006001a.
- Ewing MR, Martin H. Disability following “radical neck dissection”: an assessment based on the postoperative evaluation of 100 patients. Cancer. 1952;5(5):873-883. doi:10.1002/1097-0142(195209)5:5<873::AID-CNCR2820050504>3.0.CO;2-4.
- Buckley JG, Feber T. Surgical treatment of cervical node metastases from squamous carcinoma of the upper aerodigestive tract: evaluation of the evidence for modifications of neck dissection. Head Neck. 2001;23(10):907-915. doi:10.1002/hed.1131.
- Braverman LE, Cooper DS, eds. Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2013.
- Caron NR, Tan YY, Ogilvie JB, et al. Selective modified radical neck dissection for papillary thyroid cancer—is level I, II and V dissection always necessary? World J Surg. 2006;30(5):833-840. doi:10.1007/s00268-005-0358-5.
- Javid M, Graham E, Malinowski J, et al. Dissection of levels II through V is required for optimal outcomes in patients with lateral neck lymph node metastasis from papillary thyroid carcinoma. J Am Coll Surg. 2016;222(6):1066-1073. doi:10.1016/j.jamcollsurg.2016.02.006.
- Rao V, Subash A, Sinha P, Chatterjee S, Nayar RC. The X-pointer: a forgotten anatomical relationship of spinal accessory nerve and great auricular nerve. Surg Oncol. 2021;37:101522. doi:10.1016/j.suronc.2021.101522.
-
Anehosur V, Kulkarni K, Kumar N. Variations in the anatomy of spinal accessory nerve and its landmarks for identification in neck dissection: a clinical study. J Maxillofac Oral Surg. 2021 Sep;20(3):426-431. doi:10.1007/s12663-021-01542-z.


